Reduced Graphene Oxide – Zinc Oxide Nanocomposite Synthesis – rGO-ZnO by using Graphene Oxide, Zinc Acetate and Hydrazine Hydrate via Chemical Reduction Method
Cite This in Your Publication
Reduced Graphene Oxide – Zinc Oxide Nanocomposite Synthesis – rGO-ZnO by using Graphene Oxide, Zinc Acetate and Hydrazine Hydrate via Chemical Reduction Method - InstaNANO. https://instanano.com/all/nanomaterial-synthesis/nanocomposite/rgo-zno-1/ (accessed April 16th, 2024).
Reduced Graphene Oxide – Zinc Oxide Nanocomposite Synthesis – rGO-ZnO by using Graphene Oxide, Zinc Acetate and Hydrazine Hydrate via Chemical Reduction Method - InstaNANO. https://instanano.com/all/nanomaterial-synthesis/nanocomposite/rgo-zno-1/ (accessed April 16th, 2024).
Reduced Graphene Oxide – Zinc Oxide (rGO-ZnO) Nanocomposite Synthesis by using Graphene Oxide as Precursor, Zinc Acetate as Precursor and Hydrazine Hydrate as Reducing Agent via Chemical Reduction Method
-
CHECK LISTGraphene Oxide (GO), Zinc Acetate (Or Zinc Nitrate), Sodium Hydroxide, Hydrazine Hydrate, Deionized Water, Sonicator, Magnetic Stirrer, RB Flask.
-
STEP 1.Add 500mg Graphene Oxide in 150ml deionized water and sonicate for 40 minutes.
-
STEP 2.Add 4g Sodium Hydroxide in 100ml deionized water.
-
STEP 3.Add Sodium Hydroxide solution (prepared in step-2) in GO solution (prepared in step-1) and sonicate again for 20 minutes.
-
STEP 4.Add 2g Zinc Acetate in 100ml deionized water.
-
STEP 5.Add Zinc Acetate solution (prepared in step-4) in GO solution solution (prepared in step-3).
-
STEP 6.Add 3ml (of 10%) Hydrazine Hydrate and setup reflux at 80ºC for 24 hours.
-
STEP 7.After 24 hours remove the reflux; filter out the rGO/ZnO composite; and wash with deionized water several times.
-
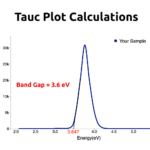
RESULTSReduced Graphene Oxide colour would be black; Raman spectroscopy will show a peak around 440cm-1. You will also get a peak around 380nm in UV-Vis Spectrosocpy.
-
Factors Affecting SynthesisConcentration of Graphene Oxide: The first main ingredient for the synthesis of rGO/ZnO composite is Graphene Oxide. As much concentration we take initially, that much amount rGO we get at the end of the process. But much higher amount of Graphene Oxide can leads to incomplete reduction and thus bad quality final product.
Concentration of Zinc Precursor: Second main salt in this synthesis is Zinc Acetate (Or Zinc Nitrate). If concentrate of Zinc Acetate is increased, then amount of reducing agent (i.e. Hydrazine Hydrate) should also be increased. Much higher concentration of Zinc Acetate will lead to bigger particles of Zinc Oxide in the final composite.
Concentration of Hydrazine Hydrate: Hydrazine Hydrate acts as the reducing agent in synthesis method. For the optimum reduction of Graphene Oxide and Zinc Acetate, optimum quantity of Hydrazine Hydrate is properly needed.
Effect of Temperature: Temperature plays an important role in speeding up any reaction. High temperature synthesis give us fine product quality by consuming less time.
Why Reflux: Reflux is a very good technique, when we want to give temperature to the solvents just below their boiling point. In this synthesis method, if we do not use reflux and direct heat the solution for 24 hours. Then all the water will be evaporated within few hours and only solid non-reduced GO will be left in the RB flask. -
NOTE: All the experiments should be done under the guidance of lab Incharge; and proper lab safety instructions.