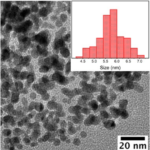
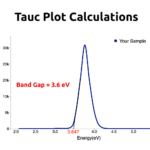
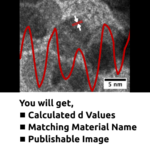
A group of humans ready to tackle world issues with fine chemistry.

I am pleased with their work. They are very excellent in their respective fields and trustful.

Great service. I managed to get my reference data from them. Really good service

One and only best website for researchers who wants their analysis to be done accurate and faster. Response was so satisfactory and highly recommended.

With amazing job and fast response and the honesty to give you all the feed back. I really recommend this website for and characterization analysis

One of the best instrumental analysis webpage where I got a lot of information, specially I got XRD analysis, matching and so on. Good Job for scientific world.

Very helpful information and this is easiest way to understand the role of plants to produce nanostructure

Great Platform for Researcher learning & guidelines.

Reply from these person is very fast and usefull. Thanks a lot for their response.

These guys are awesome and genuine …! My work was completed so fast … and result was very elaborative ..!